Sinovac-CoronaVac COVID-19 vaccine
北京科兴克尔来福疫苗 (灭活疫苗)
灭活疫苗:Inactivated vaccine
The WHO Strategic Advisory Group of Experts on Immunization (SAGE) has issued updated interim policy recommendations for the use of the Sinovac-CoronaVac vaccine against COVID-19. This article provides a summary of those interim recommendations; you may access the full guidance document here. (who.int)
世卫组织免疫战略咨询专家组(SAGE)发布了针对COVID-19使用Sinovac-CoronaVac疫苗的最新临时政策建议。本文概述了这些临时建议;您可以在这里访问完整的指导文件。(who. int)
Four vaccines are currently approved for use in the EU: Pfizer-BioNTech, Moderna, AstraZeneca and Johnson & Johnson. Another four are under “rolling review” for possible approval: Russia’s Sputnik, China’s Sinovac, Germany’s CureVac and Novavax of the United States. (straitstimes.com/July 01, 2021)
目前有四种疫苗被批准在欧盟使用:辉瑞biontech、Moderna、阿斯利康和强生。另有四家正在接受“审核”,以获得可能的批准: 俄罗斯的Sputnik,中国的科兴(China’s Sinovac),德国的CureVac和美国的Novavax。(straitstimes.com/July 01, 2021)
Omicron Covid-19 vaccine (Vero Cell ) inactivated
奥密克戎株株新型冠状病毒灭活疫苗(Vero细胞)
中国医药集团有限公司研发的疫苗
新冠疫苗加强针: Booster Shots and Additional Doses for COVID-19 Vaccines
A Study to Evaluate the Safety and Immunogenicity of Omicron COVID-19 Vaccine (Vero Cell), Inactivated in Population 18 Years Old of Age and Above (COVID-19). This is a non-randomized, open-label, externally controlled study to evaluate the safety and immnunogenicity of the Omicron COVID-19 Vaccine (Vero Cell), Inactivated in population aged 18 years old and above with no vaccination history of the COVID-19 vaccine. ( from https://clinicaltrials.gov/)
18岁及以上人群新冠奥密克戎株株新型冠状病毒灭活疫苗(Vero Cell)的安全性和免疫原性评价研究。这是一项非随机、开放标签、外部对照研究,旨在评估奥密克戎株株新型冠状病毒灭活疫苗(Vero细胞)在18岁及以上无新冠疫苗接种史人群中的安全性和免疫原性。(来自https://clinicaltrials.gov/)
Sinopharm COVID-19 vaccine (Beijing)
国药北京所新冠疫苗(灭活疫苗)
Another vaccine developed by a Beijing-based institute linked to Sinopharm, which this month obtained emergency use approval by the World Health Organization (WHO), showed a 78.1% efficacy, the paper said. The readings were based on calculations over 142 symptomatic cases in a trial involving more than 40,000 participants, with 26 injected with the Wuhan unit’s vaccine and 21 with the Beijing unit’s shot, it said. (BBC/May 27, 2021)
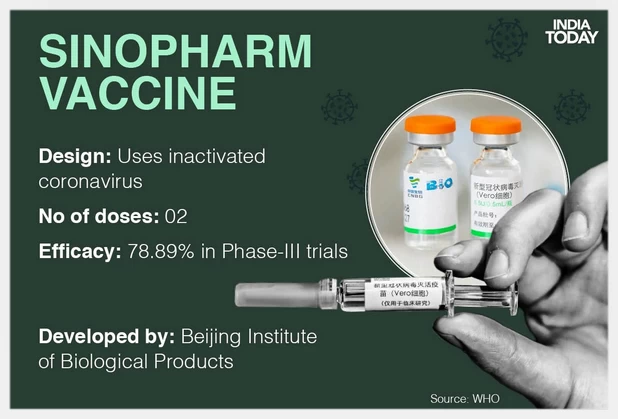
报道称,另一种由国药控股下属的北京研究所开发的疫苗,本月获得了世界卫生组织(WHO)的紧急使用批准,显示出78.1%的有效性。声明称,这些数据是基于一项涉及4万多名参与者的试验中142例有症状病例的计算得出的,其中26例注射了武汉公司生产的疫苗,21例注射了北京公司生产的疫苗。(BBC/May 27, 2021)
Sinopharm COVID-19 vaccine (Wuhan)
国药中生武汉公司新型冠状疫苗(灭活疫苗)
A vaccine developed by a Wuhan-based subsidiary of Sinopharm was 72.8% effective against symptomatic COVID-19 at least two weeks after second injection, based on interim results, the peer-reviewed study published in the Journal of the American Medical Association showed on Wednesday. (reuters.com/May 27, 2021)
周三发表在《美国医学会杂志》上的一项同行评议研究显示,根据中期结果,国药控股武汉子公司开发的一种疫苗在第二次注射后至少两周内对有症状的COVID-19有效率为72.8%。(reuters.com/May 27,2021)
CanSinoBIO COVID-19 vaccine
康希诺生物新冠疫苗(腺病毒载体新冠疫苗)
腺病毒载体新冠疫苗:adenovirus-vectored vaccine
CanSino Biologics, often abbreviated as CanSinoBIO, is a Chinese vaccine company. This Chinese company has developed a COVID vaccine based on an adenovirus in partnership with the Chinese Academy of Military Medical Sciences. The adenovirus is unable to cause disease itself, but is used to deliver a coronavirus protein. (theconversation.com/)

康希诺生物(通常缩写为CanSinoBIO)是一家中国疫苗公司。这家中国公司与中国军事医学科学院合作,开发了基于腺病毒的新冠肺炎疫苗。腺病毒本身不能引起疾病,但用于传递冠状病毒蛋白。(theconversation.com/)
Zifivax COVID-19 vaccine
安徽智飞新冠疫苗(重组新型冠状病毒疫苗/CHO细胞)
重组新型冠状病毒疫苗:Protein subunit vaccines
Chinese-based company, Anhui Zhifei Longcom, has developed a protein subunit COVID-19 vaccine known as Zifivax COVID-19 vaccine. Subunit vaccines use a purified piece of the virus, a protein, to trigger an immune response. It has recently started phase 3 clinical trials. There hasn’t yet been any announcement or published report of the results of phase 1 and 2 trials. (theconversation.com/)
中国安徽智飞公司开发了一种新型冠状病毒蛋白亚单位疫苗。亚单位疫苗使用纯化的病毒片段(一种蛋白质)来触发免疫反应。该公司最近开始了三期临床试验。目前还没有任何关于1期和2期试验结果的公告或发表报告。(theconversation.com/)
Indonesia has approved a COVID-19 vaccine produced by Chinese company Anhui Zhifei Longcom Biopharmaceutical for emergency use, the country’s food and drug agency BPOM said on Thursday.
“Today, BPOM again announces that we have approved a COVID-19 vaccine product under the trade name Zifivax, which was developed with a recombinant protein sub-unit platform,” BPOM’s head Penny Lukito told a virtual press conference. (news.cgtn.com)
印尼食品药品监督管理局(BPOM)周四表示,印尼已批准中国安徽智飞生物制药公司生产的COVID-19疫苗用于紧急使用。
BPOM负责人Penny Lukito在虚拟新闻发布会上表示:“今天,BPOM再次宣布,我们已经批准了一种商品名为Zifivax的COVID-19疫苗产品,该产品采用重组蛋白亚单位平台开发。”(news.cgtn.com)
相关报道
各类疫苗效果
张文宏称,根据团队的Meta分析,从整体上来看,灭活疫苗(Inactivated vaccine)的保护率为73.11%,腺病毒载体疫苗(adenovirus-vectored vaccine)的保护率为79.56%,亚单位疫苗(protein subunit vaccines)的保护率为89.33%,mRNA疫苗(美国)的保护率为94.29%。(每日经济新闻 2023-01-03)
各类疫苗需要第几针?
综上,中国新冠疫苗品牌有国药中生北京公司的灭活疫苗、北京科兴中纬公司的灭活疫苗、国药中生武汉生物制品的灭活疫苗、智飞生物重组新冠疫苗(CHO细胞)、天津康希诺腺病毒载体疫苗。而它们之间最大的区别就是灭活疫苗只需打两针,重组新冠疫苗需要打三针,腺病毒载体疫苗只需要打一针,其它安全和有效并无很大的区别。(愽禾医生/2022-01-18)
